Understanding the Specific Heat of Iron: Key Facts You Need to Know

The specific heat of iron is an important concept to understand if you’re diving into material science or physics. This property tells us how much energy is required to raise the temperature of a certain amount of iron by one degree. Knowing the specific heat of iron helps in many practical applications, like designing machines or understanding how heat travels through metal.
When we talk about the specific heat of iron, we are looking at the energy needed to change the temperature of the material. Iron, being a common metal used in construction and engineering, has a specific heat capacity that affects how it responds to heat. This article will explore what the specific heat of iron is, why it matters, and how it compares to other metals.
What is the Specific Heat of Iron? Understanding the Basics
The specific heat of iron tells us how much energy is needed to increase the temperature of a given amount of iron by one degree. When a metal like iron is heated, its molecules move faster. The amount of heat required to cause this temperature change depends on the specific heat of the material. For iron, this value is around 0.45 J/g·K (Joules per gram per Kelvin). This means it takes 0.45 joules of energy to raise the temperature of one gram of iron by one degree.
Understanding this concept helps in many industries, from building bridges to creating machines. It is essential for engineers to know how much heat a material will store and release. Iron is commonly used because of its properties, and its specific heat capacity makes it a reliable material for many applications, like engines and buildings.
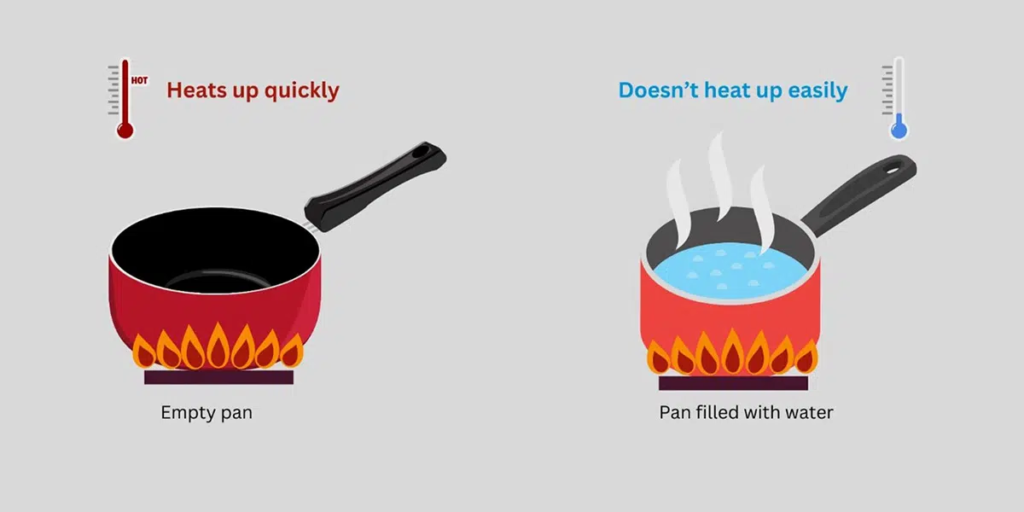
Knowing the specific heat of iron is also useful in everyday life. When you heat up a pot or pan, the material it’s made of will influence how quickly it gets hot. Iron’s specific heat explains why it takes a bit longer for an iron pot to heat up compared to other metals. Its ability to hold heat makes it a perfect choice for cooking and other applications.
How the Specific Heat of Iron Affects Heat Transfer in Everyday Objects
The specific heat of iron plays a significant role in how heat is transferred in objects made of iron. Heat transfer is the process by which heat moves from one place to another. When iron heats up, it takes longer for it to lose that heat, thanks to its specific heat. This means objects like iron pans retain heat for a long time, which is perfect for cooking or keeping things warm.
In objects like radiators or engines, the ability of iron to store and release heat affects how they work. Iron engines, for instance, benefit from this property because the heat doesn’t escape too quickly. It helps maintain steady temperature levels, which is crucial for the performance and efficiency of the machine.
Comparing the Specific Heat of Iron with Other Metals: A Quick Guide
When we compare the specific heat of iron with other metals, we find that iron has a moderate specific heat. It’s not too high, like water, nor too low, like lead. Iron’s specific heat allows it to heat up and cool down at a balanced rate, making it useful for various purposes.
Metals and their Specific Heat:
- Iron: 0.45 J/g·K
- Copper: 0.39 J/g·K (Copper heats up faster than iron)
- Aluminum: 0.90 J/g·K (Aluminum holds more heat than iron)
- Lead: 0.13 J/g·K (Lead holds less heat than iron)
Why Knowing the Specific Heat of Iron is Crucial for Engineers
For engineers, understanding the specific heat of iron is key to making effective designs. Whether it’s in construction or machinery, the way iron reacts to heat can change the overall performance of the project. By knowing how much heat iron will store and release, engineers can predict how materials will behave under different temperatures.
In the world of machinery, for instance, iron’s heat retention is useful in engines. It allows them to maintain steady operating temperatures, preventing overheating. This makes iron a popular choice for parts that need to withstand high temperatures for long periods, like engine blocks and brake systems.
In construction, the specific heat of iron also plays a role in buildings, bridges, and railways. Knowing how iron will respond to heat helps engineers make sure structures remain safe and stable. For example, iron beams in a bridge must be able to handle heat without expanding too much, which could lead to damage.
The Role of Specific Heat of Iron in Metal Manufacturing Processes
The specific heat of iron is also important in manufacturing. When iron is melted or shaped into different products, it needs to be heated to very high temperatures. The way it absorbs and releases heat affects how easily it can be manipulated. For instance, in steel-making, the specific heat determines how much energy is needed to melt iron and create steel.
In factories, machines rely on the specific heat of iron to control temperature during production. Heating and cooling must be done carefully to avoid damaging the iron. If the heat is applied too quickly, it might cause the metal to crack. If it’s too slow, the production process might take longer than necessary.
Manufacturers also use the specific heat to determine how to best cool iron after it has been melted. The slower cooling process can produce stronger, more durable iron, which is why understanding its heat properties is so crucial.
The Effect of Alloying on the Specific Heat of Iron: What You Should Know
When iron is combined with other metals to form alloys, its specific heat can change. Alloys are made by mixing iron with metals like carbon, nickel, or chromium. These changes can affect the heat capacity of the material in different ways, depending on the alloy’s composition.
Common Iron Alloys and Their Specific Heat:
- Steel: The specific heat of steel, an iron-carbon alloy, is slightly lower than pure iron. This makes steel a more efficient material for certain applications.
- Stainless Steel: A mix of iron, chromium, and nickel, stainless steel has a slightly higher specific heat compared to regular steel, making it better at withstanding heat without degrading.
- Cast Iron: This alloy has a similar specific heat to that of pure iron but is much stronger and can handle higher temperatures.
Comparing the Specific Heat of Iron with Other Metals

When comparing the specific heat of iron with other common metals, it becomes clear that different metals respond to heat in different ways. Iron’s specific heat is around 0.45 J/g·K, which is moderate compared to other metals. Some metals heat up faster, while others retain heat for longer periods.
For example, copper has a specific heat of 0.39 J/g·K, which means it heats up slightly faster than iron but doesn’t retain heat as well. Aluminum, with a higher specific heat of 0.90 J/g·K, can hold more heat and takes longer to warm up. Lead, with a specific heat of only 0.13 J/g·K, has one of the lowest heat capacities, meaning it heats up and cools down much faster than iron.
These differences in specific heat are important when choosing materials for various applications. For example, aluminum might be preferred in cooking pots for its quicker heat distribution, while iron might be chosen for its ability to retain heat over a longer period. Similarly, in construction and machinery, the specific heat of iron may make it a better choice for parts that need to manage heat over time.
Conclusion
The specific heat of iron is an important property that helps us understand how iron reacts to heat. Whether in cooking, machinery, or construction, knowing how much heat iron can hold and how it responds to temperature changes is useful in many situations. Iron’s moderate specific heat makes it a versatile material for many everyday items, and engineers rely on this knowledge to make sure iron-based products work safely and efficiently.
Understanding the specific heat of iron also helps in comparing it with other metals, allowing us to choose the right material for specific tasks. From cooking pots to complex machinery, the ability of iron to store heat for a longer time gives it a special role in many industries. With the right knowledge, we can use iron more effectively and keep things running smoothly.
FAQs
Q: What is the specific heat of iron?
A: The specific heat of iron is 0.45 J/g·K, which means it takes 0.45 joules to raise the temperature of one gram of iron by one degree Celsius.
Q: Why is the specific heat of iron important?
A: The specific heat of iron helps engineers and manufacturers know how the material will react to temperature changes, making it safer and more efficient in many uses.
Q: Does iron heat up quickly?
A: No, iron doesn’t heat up as quickly as metals like copper. It heats up at a moderate rate due to its specific heat value.
Q: How does the specific heat of iron affect cookware?
A: Iron retains heat for a long time, which is why iron cookware like pans and skillets stay hot for longer, making them great for cooking.
Q: How does the specific heat of iron compare to other metals?
A: Iron has a moderate specific heat, meaning it heats up and cools down at a balanced rate compared to metals like copper and aluminum, which behave differently.